Transgenic plants are plants that have had their genomes modified through genetic engineering techniques either by the addition of a foreign gene or removal of a certain detrimental gene. A foreign gene inserted into a plant can be of a different species or even kingdom. The first transgenic plant was developed through the insertion of the nptII bacterial antibiotic resistance gene into tobacco. Since then, with the rapid development in plant molecular biology and genetic engineering technology, a wide variety of transgenic plants with important agronomic traits such as pest resistance and drought tolerance have been developed, ranging from dicots to monocots that are amenable to genetic modifications. The main purpose of the production of transgenic plants is to produce crops, which have ideal traits, quality, and high yield. Besides being beneficial to the agriculture sector, the plants are found to be able to act as the factory for pharmaceutical protein production.
Biotic stresses occur naturally as a result of stress exerted from other living organisms within the same ecosystem. These include bacteria, viruses, herbivores, or native plants.
Malnutrition is a major health concern that is prevalent especially in the underdeveloped and developing countries due to limited access to nutritious food. Genetic engineering of staple crops has become one of the more effective solutions in addressing this problem.
Product yields from recombinant proteins using mammalian expression systems are low and expensive, while the bacteria system is incapable of post-translational modification in complex protein formation. Due to this, the production methods had shifted to plant cell systems, which provide cheaper and better alternative sources for recombinant protein production. The recombinant proteins produced in transgenic plants include antibodies, metabolites or catabolites, proteins, and vaccines.

A simple functional gene construct consists of a promoter region, gene coding region, and terminator/stop region. In addition, certain gene constructs may contain special sequences such as an enhancer, silencer, or reporter sequences depending on the nature of the study. Plant transformation always starts with the transgene construction. Transgene construct generally has similar elements other than the inclusion of the gene of interest and selectable markers. A proper gene construct is crucial for the success of producing an ideal transgenic line.
(1)A typical plant gene
(2)Promoters/enhancers
Table 1 shows selected promoters used in plant transformation.
|
Promoter |
Source |
Activity |
|
CaMV35S |
Cauliflower mosaic virus |
Constitutive |
|
Ubiquitin RUBQ1, RUBQ2 and rubi3 |
Rice |
Constitutive |
|
Ubiquitin Gmubi3 |
Soybean |
Constitutive |
|
SCR, SRK |
Brassica rapa |
Pollen and stigma specific |
|
Exo70C2 |
Arabidopsis |
Pollen and root specific |
|
LMW Glu, HMW Glu-1D1 |
Wheat |
Seed specific |
|
Expansin PcExp2 |
Sour cherry |
Ripened fruits |
|
Potato class I patatin |
Potato |
Tuber/storage organ specific |
|
NtHSP3A |
Tobacco |
Stress inducible |
Table 1.
Examples of promoter used in plant transformation.
Source: Adapted from Hernandez-Garcia et al.
Enhancers are short (50–1500 bp) regions in a gene that can be recognized and bound by activator proteins. These proteins, also referred to as transcription factors, bind to the enhancer, forming an enhancer-bound transcription factor complex, which will later on interact with the mediator complex (TFIID) ultimately aiding in the recruitment of RNA polymerase II.
Reporter genes are genes attached to the regulatory sequences or to the gene of interest to allow for the detection of the transgene expression as well as the localization of expressed proteins. Reporter gene sequences encode proteins or products of the protein after being catalyzed for detection through instruments or simple assays. In contrast, selectable marker genes such as antibiotic genes, herbicidal-resistant genes, and anti-metabolic genes confer resistance toward certain chemical agents, which inhibit nontransgenic plant development.
Plant transformation techniques available currently are rather efficient but not perfect yet. There are no techniques that are able to provide 100% transformation efficiency. In order to distinguish the transformed and nontransformed plant cells, markers are needed. Antibiotic or herbicide resistance genes act as the primary selective markers in transformant selection to efficiently eliminate the nontransformants. The effectiveness of an antibiotic resistance system is dependent on three criteria:
(1) selective agent used should completely inhibit the growth of nontransformed cells,
(2) resistance gene is expressed in transformed cells,
(3) explant used for transformation.
Table 2 shows some of the antibiotics used in transgenic plant screening.
|
Antibiotics |
Mechanism of action |
General working concentration (μg/ml) |
Selection |
|
Kanamycin |
Inhibiting ribosomal translocation and eliciting miscoding |
50 |
nptII |
|
Hygromycin B |
Inhibit protein synthesis |
20–200 |
hph |
|
Streptomycin |
Inhibit protein synthesis |
100 |
spt |
|
Spectinomycin |
Inhibit protein synthesis |
100 |
aadA |
|
Phleomycin |
DNA breakage |
10 |
ble |
|
Bleomycin |
DNA breakage |
10 |
ble |
Selective antibiotics used for transgenic plants screening.
A vector acts as a vehicle that transports the gene of interest into a target cell for replication and expression. The Common vector consists of three components: an origin of replication, a multi cloning site or a recombination site, and a selectable marker.
The Ti plasmid is large and would become larger with the genes of interest and selectable markers. Large-sized plasmids are cumbersome to handle and have low copy numbers in nature. However, this drawback eventually led to the development of a co-integrative system in combination with the binary vector system which solved the problem for large-sized plasmids.
This co-integrative vector will later be introduced back into the Agrobacterium for transgenic plant transformation. However, the enormous size of the plasmid as a result of the recombination may prove an ominous challenge to be manipulated. Thus, the use of this vector had been discontinued since the binary vector system was introduced.
A two-plasmid system called the binary vector system was developed when researchers found that T-DNA functioned independently without the need to attach to the Ti plasmid. The binary system involved two plasmids which are the helper vector and mini vector. The mini vector refers to a smaller size plasmid consisting of the T-DNA and the origin of replication of both E. coli and A. tumefaciens, which allow the plasmid to be cloned in E. coli and A. tumefaciens. The helper vector refers to a wild-type Ti plasmid without the T-DNA region. The wild-type Ti plasmid is also known as a helper plasmid as it provides the template for all the genes necessary for gene transferring and integration. Both of these helpers and mini vectors are introduced together into the Agrobacterium and the transformed Agrobacterium will be used in plant transformation.
Viruses are intracellular obligate parasites that require molecular machinery from a specific host to replicate. Viruses have not been found to infect plants through the use of transmission vectors such as aphids, insects, nematodes, and fungi. These viruses have been modified and are used as alternative sources for plant transformation; common plant viruses used in transgenic plant production include the Cauliflower mosaic virus (CaMV), Tobacco mosaic virus (TMV), Alfafa mosaic virus (AMV), Potato virus X (PVX), and Cowpea mosaic virus (CPMV). The wild-type plant viral vectors have been improved and modified to accommodate their use with Agrobacteria as well as the plant host for an increased efficiency level through two approaches. The first approach would be designing virus vectors that are similar to wild types carrying the gene of interest, which are capable of infecting plants.
The second approach would be the development of a ‘deconstruct’ virus, which occurs through the removal of the undesired viral genes, for example, the coat protein-expressing gene, and to replace them with functional genes such as reporter genes or antibiotic resistance gene, which facilitates transgenic screening.
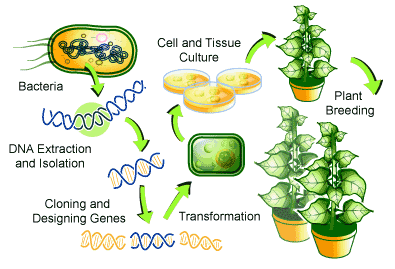
Plant transformation refers to the process of altering the genetic constituents in a plant of interest by introducing DNA segments into the plant genome to achieve desired gene expression. Numerous types of plant transformation techniques have now been made accessible to the public. These plant transformation techniques can be categorized into two groups: indirect or direct gene transfer. Indirect gene transfer (also known as vector-mediated gene transfer) involves the introduction of exogenous DNA into the plant genome via biological vectors, whereas direct gene transfer methods involve the introduction of exogenous DNA directly into the plant genome through physical or chemical reactions. Different gene transfer methods and their salient features are tabulated in Table 3.
|
Method |
Features |
|
Vector-mediated gene transfer |
|
|
a. Agrobacterium-mediated gene transfer |
Efficient to wide range of plants. |
|
b. Plant virus vectors |
Efficient and high expression of transgenes. |
|
Direct gene transfer |
|
|
1. Electroporation |
Confined to protoplasts that can be regenerated to produce complete and viable plants. |
|
1. ii. Microinjection |
Requires highly skillful technical personnel and limited to one cell per microinjection. |
|
1. iii. Particle bombardment/microprojectile |
Special instrumentation required. High risk of gene rearrangement. May be used for a wide range of plant tissues. |
|
1. iv. Silicon carbide fibers |
Requires careful handling. Requires regenerable cell suspensions. |
|
b. Chemical methods |
|
|
1. v. Polyethylene glycol (PEG)-mediated |
Confined to protoplasts. Problems encountered when regenerating these cells into viable plants. |
|
1. vi. Liposome fusion |
Confined to protoplasts which may be regenerated into a viable plant. |
|
1. vii. Diethylaminoethyl (DEAE) dextran mediated |
Does not result in stable transformation. |
Table 3.
Gene transfer methods in plants and their features.
Agrobacterium-mediated transformation is the most common technique used in plant transformation as it is efficient and effective in a wide range of plants.
Direct gene transfer, as the name suggests, involves the direct introduction of exogenous DNA (naked DNA) into the plant nucleus. In order to introduce foreign DNA into the plant cell, the outer membrane of the cell is first disrupted, permeating it for foreign DNA to enter. Most of the methods under direct gene transfer are simple and effective. However, gene expression in these transgenic plants can be transiently or stably transformed.
The genetic seints of plant genetic information usually follow the Mondelli genetic law. Mendel's first law, the separation principle, states that a pair of alleles of each gene will be separated during the formation of the ligand, resulting in each matching containing only one gene allele. Mendel also found that in the formation of matching, genes with different characteristics are independent of each other; In general, genetic models are usually analyzed by the separation of molecular characterization and genetically modified phenotype expression patterns transmitted by the transgene.
|
|
Factors |
|
Nature of recipient genome |
Genetic background |
|
Nature of transgene |
Transgene silencing |
|
Interactions between the recipient genome and the transgene |
Homozygous lethality |
Table 4.
Factors leading to non-Mendelian inheritance of transgene.
Source: Adapted from Yin et al.
The analysis and validation of transgene integration must be done in an appropriate way based on transgene structures, optional marking and reporting of genes. GM plant cells bind to anti-hemophilia or antibiotic-resistant genes and are screened by adding herbicides or antibiotics to the growth medium to distinguish between converted plant cells and non-transformed plant cells. However, this method requires a large number of antibiotics and herbicides, which are expensive and worsen due to the risk of horizontal gene transfer to other bacteria. Therefore, other screening methods, such as polymerase chain reaction (PCR) and reporting gene expression screening, are more accurate as an alternative screening method for genetically modified plants.
Some reported genes, such as GFP, GUS, and Luc expressions, are fluorescent or chroma, where the expression of these genes can be observed visually or directly under a microscope. The reporter expression can be quantified using a spectrophotometer. GUS expression can also be detected by tissue chemical assay, in which the positioning of genetically modified instruments can be observed. In addition, some reported gene expression, such as CAT and LacZ activity, were screened by enzyme testing.
The polymerase chain reaction (PCR) method is one of the most sensitive and simplest of all molecular techniques used for transgene validation. PCR is usually done with primers specific to the plasmid level and genes of interest for the development of genetically modified plants. The expected band successfully amplified DNA fragments to indicate the possibility of transgene, and the DNA fragments were further confirmed by DNA sequencing. Compared with traditional southern spot analysis, real-time PCR provides fast, sensitive and high-volume molecular PCR analysis, especially in the field of transgene copy number and enzyme detection of genetically modified plants. Real-time PCR is convenient and allows quantitative, semi-quantitative (qPCR) or qualitative (RT-qPCR) real-time monitoring of the target DNA.
GM crops will be a valuable option to address food security in a world of growing populations and climate change. However, gmability remains a major controversy because public misunderstandings and perceptions lead to biosecurity problems in biological plants.
In addition, GM crops require years of risk assessment, which is time-consuming and cost-effective. On the other hand, the emergence of unexpected effects may be one of the problems in the production of GM plants. This is usually due to the integration of GM through illegal recombination in plants as a result of random GM integration, genetic destruction, sequence changes and the production of new proteins. Therefore, the unexpected effects of gene transfer in GM crops should be thoroughly studied through metabolic analysis methods to avoid the production of genetically modified plants with significant differences in chemical composition from non-GM plants grown under the same conditions.
Recently, engineered site-specific endoenzymes, such as TFN, TALEN, and CRISPR/Cas9, have been developed to enable plant genetic engineering to be performed more efficiently and accurately. The problems of miscellaneous common in agriculture and gene gun-tossing methods can be avoided. Therefore, the future of GM technology is shifting to the engineering subenzyme genome editing technology. This endogenous genome editing involves the introduction of targeted double-stranded DNA fracture (DSB) into the genome to stimulate cellular DNA repair mechanisms. In addition, different genomic modifications can be performed depending on the type of DSB repair pathway used: (1) non-homogenic end join (NHEJ) and (2) homologous recombination (HR).
In NHEJ-mediated genome editing, target cells edit their genomes without adding foreign genes that could lead to mutations and gene knock-offs. Because this genome editing is done without the introduction of foreign genes, non-GMO crops can be obtained. Therefore, there is a need to work together to improve genomic editing techniques for genetically engineered crops with better agronomic characteristics and public acceptance.
Related Services
Microorganisms Gene Modification Services
Contact Information
Please obtain a quote before ordering, and refer to the quote number when you place an order.
Orders are typically confirmed within 12 hours.
Have a Question? Email us info@leadingbiology.com
Order Products: Order Related Products
By Phone: 1-661-524(LBI)-0262 (USA)
| No | Headline | Click | Author | Date |
| 1 | Genome Editing: Which Should I Choose, TALEN or CRISPR? | 1642 | Leading Biology | 2020-01-03 |
| 2 | TALEN - Transcription activator-like effector nuclease | 2161 | Leading Biology | 2020-01-02 |
| 3 | Microorganisms Gene Modification Services | 1363 | Leading Biology | 2019-12-30 |
| 4 | CRISPR-CAS Technology | 1727 | Leading Biology | 2019-12-30 |
| 5 | Transgenic Plants Construction | 1659 | Leading Biology | 2019-12-30 |
| 6 | In Situ Hybridization | 2538 | Leading Biology | 2018-01-26 |